 |
|
 |
Home
Phosphate glasses
Phosphate based glasses are a special kind of materials whose composition and properties can be tailored within a very wide range. Generally, phosphate glasses posses low glass transition temperature, high coefficient of thermal expansion and high transmission to the ultraviolet wavelengths. Furthermore, their low melting temperature and special chemistry contribute to produce glasses which can host relatively high amounts of transition metal ions as well as rare earth elements. To date, many potential applications have been proposed for phosphate glasses. Their special thermal behaviour makes them suitable for low temperature sealing materials and numerous studies have been published on it. Nd-doped phosphate glasses have received special attention for their application as solid-state laser host matrices with exceptional performance. Feasibility for the application of phosphate glasses and protonic conductors, nuclear waste host matrices when containing iron or lead oxides as well as semiconducting glasses have also attracted much attention. More recently, phosphate glasses have been studied for their interest within the Biomaterials field. However, the most important factor limiting the practical application of phosphate glasses is their extremely low chemical durability. A demonstrated way to radically improve the chemical durability of phosphate glasses is based on the partial substitution of nitrogen for oxygen, firstly developed by Marchand in the early 80s.
Oxynitride glasses (Poster presentation at the 10th Glass Trends meeting, Zandvoort (The Netherlands), 2011)
The structure of phosphate glasses is built up of PO4 tetrahedra, in which at least one of the oxygens will be of the non-bridging type (NBO) due to the pentavalency of the phosphorous atom. Modifier ions are bonded to non-bridging oxygens and, depending on their concentration, phosphate glasses are then classified in ultraphosphate for O/P ratios >3, metaphosphate glasses, where all PO4 tetrahedra have two bridging and two equivalent non-bridging oxygens and polyphosphate glasses for an O/P ratio < 3. In oxynitride phosphate glasses, nitrogen substitutes for both bridging and non-bridging oxygens and nitrogen can appear in the form of dicoordinated, Nd (-N=), or tricoordinated atoms, Nt (-N<), where the Nd/Nt ratio also depends on the initial composition of the glasses. Simultaneously, new PO3N and PO2N2 tetrahedra are formed from PO4 ones. The increased bonding density and higher covalent character of the P-N bonds against P-O ones are responsible for the drastic increase in chemical durability of the glasses. It has been proposed that the dissolution mechanism changes with respect to the one operating in pure oxide phosphate glasses. The new mechanism of dissolution must involve the hydrolysis of P-N bonds, constituting a big hindrance for their dissolution.
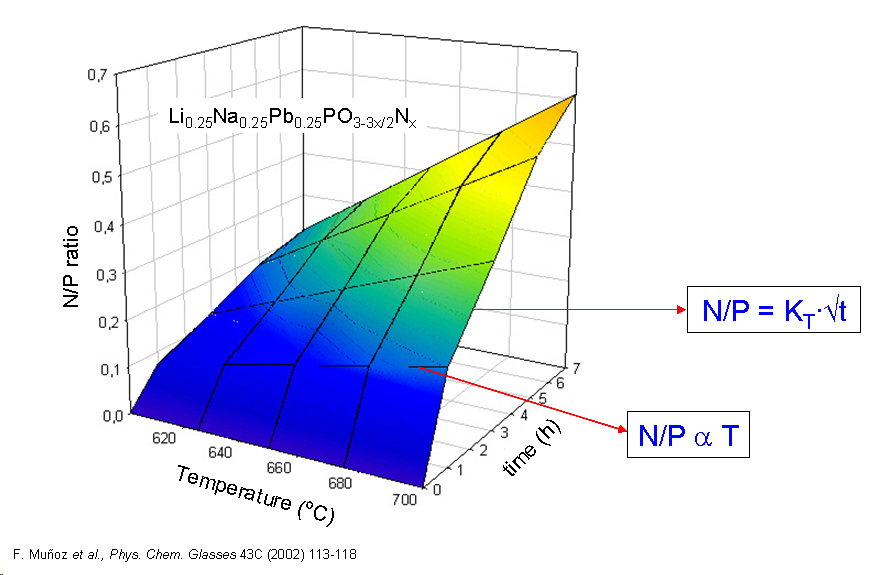
The study of oxynitride phosphate glasses has been focused on the kinetics of nitridation, the structure of the oxynitride glass network, and the influence of nitrogen on the main properties of the glasses. The introduction of nitrogen might extend the applicability of phosphate glasses and some other potential uses of oxynitrides have been proposed, like their use as all-solid-state electrolytes for lithium rechargeable batteries due to the increased electrical conductivity of the oxynitride.
Solid electrolytes
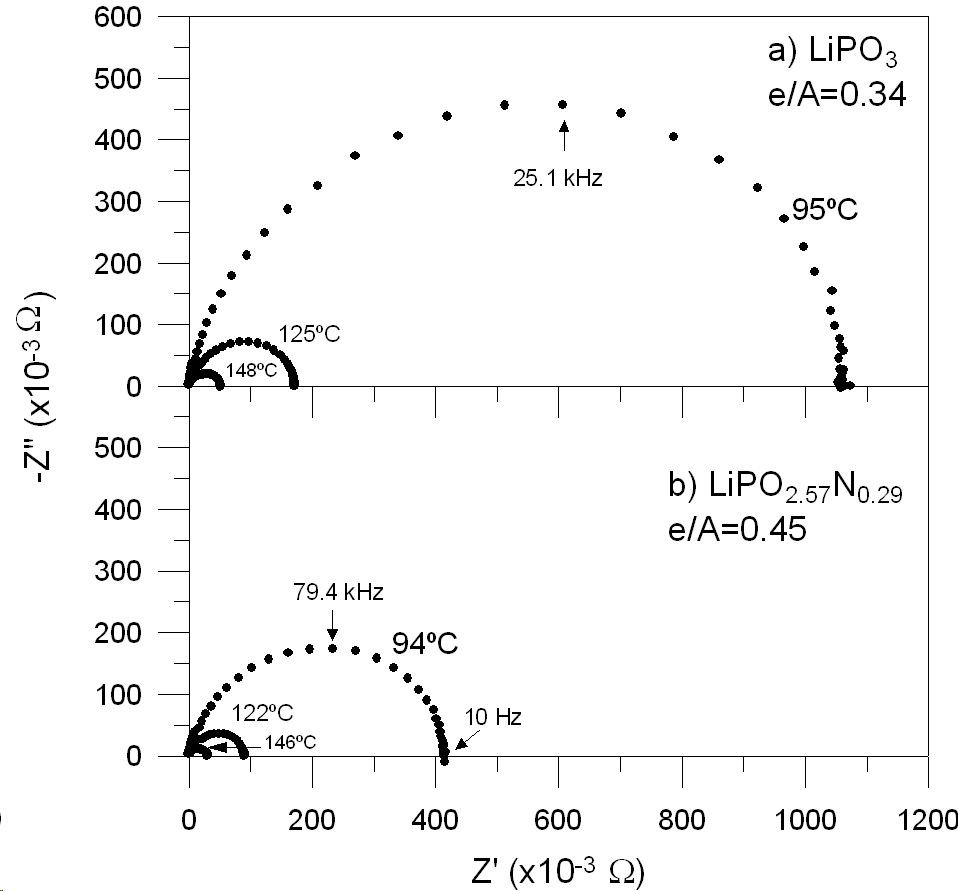
The development of glassy materials for their application in energy production has been identified as a priority research line within the field of glasses and glass-ceramic materials for energy and environment. In particular, the research of novel solid electrolytes for rechargeable lithium batteries has become a developing area which opened new ways of technological applications. The incorporation of glasses in all-solid-state devices might overcome many of the drawbacks related to the use of liquid electrolytes, such as the contamination of the electrodes or the risk of explosions. In addition, the ease of the glass processing, the great variety of compositions that can be prepared, as well as the alternative application of electrolytes in the form of glass-ceramics, make glasses highly versatile in comparison to their crystalline counterparts. The main requirements for a solid-state electrolyte are a pure ionic conductivity, high chemical and mechanical stability within the voltage ranges and compatibility with the electrode materials. The glasses possessing the highest electrical conductivities at 25şC are based on the system LiI-LiF-Li2S-Li2O-P2O5-P2S5. These glasses can reach conductivities up to 10-3 S.cm-1, although they require special synthesis conditions due to their hygroscopicity, chemical instability and volatility of their components. An alternative way of producing highly conducting electrolytes involves the preparation of glass-ceramics, in which crystalline phases with the NASICON-type structure may be devitrified, e.g. Li2O-Al2O3-GeO2-P2O5 and Li2O-Al2O3-TiO2-P2O5. Within this field, many important laboratories, as well as battery producers, are making efforts to open new ways in lithium battery research through the investigation of glasses and glass-ceramics as solid-state electrolytes. Lithium borophosphate glasses have been obtained through different routes and proposed as solid electrolytes in lithium batteries. Due to the generally low chemical durability of phosphate-based glasses, improvement of its stability has always been a major aim in glass research. The chemical durability of high Li2O-containing phosphate glasses was demonstrated to be greatly augmented by small additions of Al2O3, which at the same time, gives rise to an enhanced electrical conductivity. This research line is focused on the investigation of the binary Li2O-P2O5 system, including oxynitride compositions, and the influence of other oxides, such as SiO2, B2O3 and Al2O3, on the electrical conductivity. Special attention has been paid to the structural characterisation of the glasses, followed by Nuclear Magnetic Resonance, allowing a new interpretation of the effect of nitrogen on the electrical conductivity of lithium phosphate glasses.
|